Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine to authorize bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination. The bivalent vaccines, which […]
Read More
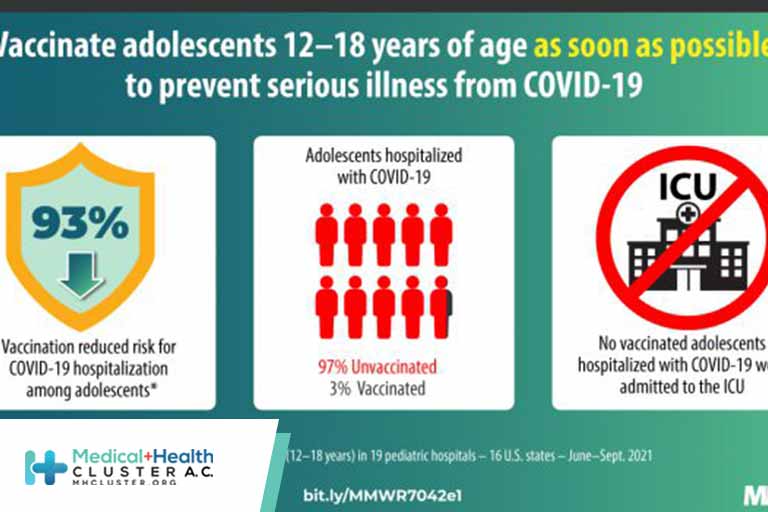
Summary What is already known about this topic? Persons aged 12–18 years are eligible to receive COVID-19 vaccine. Currently, data are lacking on real-world vaccine effectiveness against COVID-19 hospitalization in adolescents. What is added by this report? Among hospitalized U.S. patients aged 12–18 years, vaccine effectiveness of 2 doses of […]
Read More
Pfizer Inc. and BioNTech SE said a booster shot of their Covid-19 vaccine restored full protection in a large study, results that are likely to bolster the argument for giving a third dose more widely. A booster was 95.6% effective against symptomatic Covid in the study, which followed 10,000 people aged 16 and […]
Read More
Summary What is already known about this topic? Persons aged 12–18 years are eligible to receive COVID-19 vaccine. Currently, data are lacking on real-world vaccine effectiveness against COVID-19 hospitalization in adolescents. What is added by this report? Among hospitalized U.S. patients aged 12–18 years, vaccine effectiveness of 2 doses of […]
Read More
Con un número récord de casos de COVID-19 reportados en la población pediátrica, Pfizer y su socio BioNTech anunciaron que su vacuna de ARN mensajero contra la COVID-19 es segura y parece generar una respuesta inmunitaria protectora en la población de 5 años y más.[1] Las compañías han estado probando una dosis más baja de […]
Read More
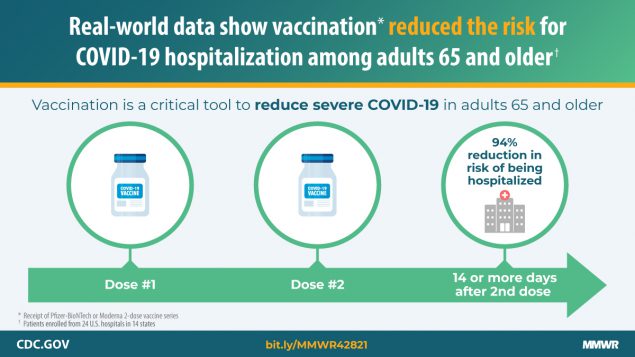
Un nuevo informe en el MMWR de los CDC está entre los primeros en evaluar los beneficios de las vacunas de ARNm contra el COVID-19 (Pfizer-BioNTech, Moderna) contra la hospitalización en condiciones de la vida real. Las personas de 65 años o más completamente vacunadas tenían un 94 % menos […]
Read More