La pandemia desencadenada por el SARS-CoV-2 ha cambiado desde aquel primer caso en Wuhan en 2019, actualmente contamos con eficientes vacunas que han permitido el regreso a nuestras actividades cotidianas. Asimismo, con el transcurso del tiempo se han posicionado tratamientos que están disponibles en mayor o menor medida para el […]
Read More
Monoclonal antibodies (mAbs) are highly effective in treating mild to moderate COVID-19 among nonhospitalized patients.1 Given limited supply, federal guidelines prioritize patients at higher risk of progression to hospitalization or mortality from COVID-19, with risk factors including age and comorbid conditions.2,3 Antibodies were initially allocated to states by the federal government,4 then distributed […]
Read More
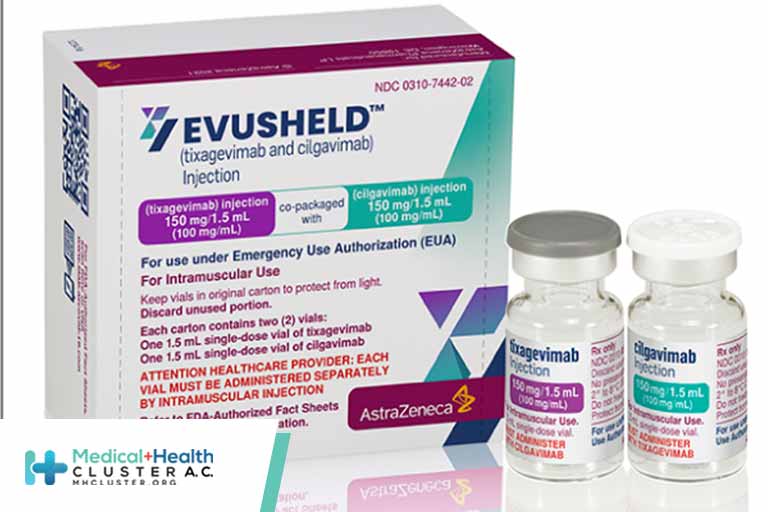
Every day for weeks this past fall, Brian Koffman, MD, would Google “AZD7442, AZD7442, AZD7442.” He finally hit pay dirt December 8, when he discovered that the US Food and Drug Administration had authorized AstraZeneca’s long-acting monoclonal antibody combination, AZD7442 (Evusheld), for COVID-19 preexposure prophylaxis in adults and children at least 12 years […]
Read More
The newly identified antibody was isolated using lymphocytes from COVID-19 patients enrolled in the ImmunoCoV study being carried out by CHUV’s Service of Immunology and Allergy. This antibody is one of the most powerful identified so far against SARS-CoV-2. Structural characterization of the antibody indicates that it binds to an […]
Read More
The FDA authorized monoclonal antibody drugs from the companies Regeneron and Eli Lilly in November 2020, but only recently have they attracted more attention as the Delta variant of the virus that causes COVID-19 surges across the U.S. Clinical trials show that Regeneron’s monoclonal antibody treatment, a combination of two […]
Read More
An injected monoclonal cocktail given to household contacts of patients with COVID-19 was 81% effective. Postexposure prophylaxis with immunoglobulins has been the standard of care for many infectious diseases; thus, an effective immunoglobulin preparation for COVID-19 would provide a powerful new public health approach to controlling the current pandemic. Researchers […]
Read More
A combination of two monoclonal antibodies given as a subcutaneous injection prevented COVID-19 in patients at a high risk of infection due to household exposure, according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4 in the New England Journal of Medicine. The cocktail of the monoclonal antibodies casirivimab and […]
Read More
Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center. JOHN WHYTE: Welcome, everyone. I’m Dr. John Whyte, Chief Medical Officer at WebMD, and you’re watching Coronavirus in Context, where we try to break down what’s happening with coronavirus and how it impacts your life. We’ve been talking a […]
Read More
Educational Impact Challenge The goal of this activity is to increase clinicians’ knowledge and understanding of how neutralizing monoclonal antibodies function to both treat patients with COVID-19 and prevent disease in others. Before you begin this activity, please assess your clinical knowledge by completing this brief survey. Answering these questions again after […]
Read More
Monoclonal antibody treatment: GlaxoSmithKline and Vir Biotechnology are seeking emergency use authorization for their investigational monoclonal antibody treatment for early COVID-19. In an interim analysis of an efficacy trial of nearly 600 adults with COVID-19 who were at high risk for hospitalization, VIR-7831 was associated with an 85% lower risk […]
Read More