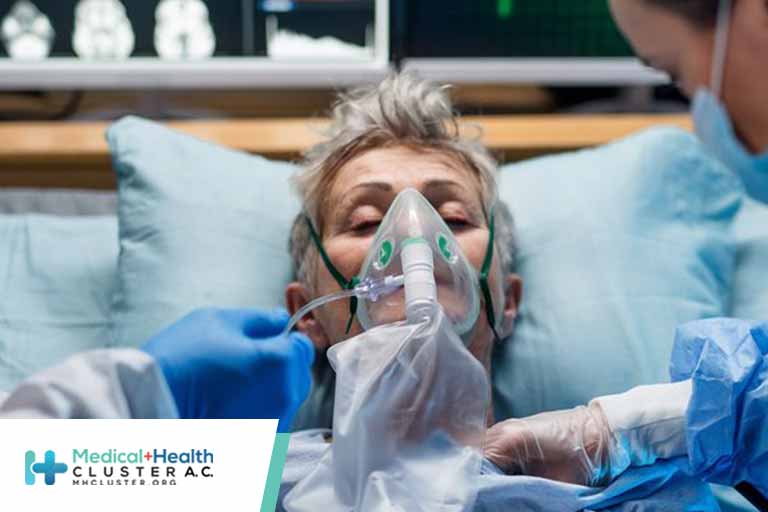
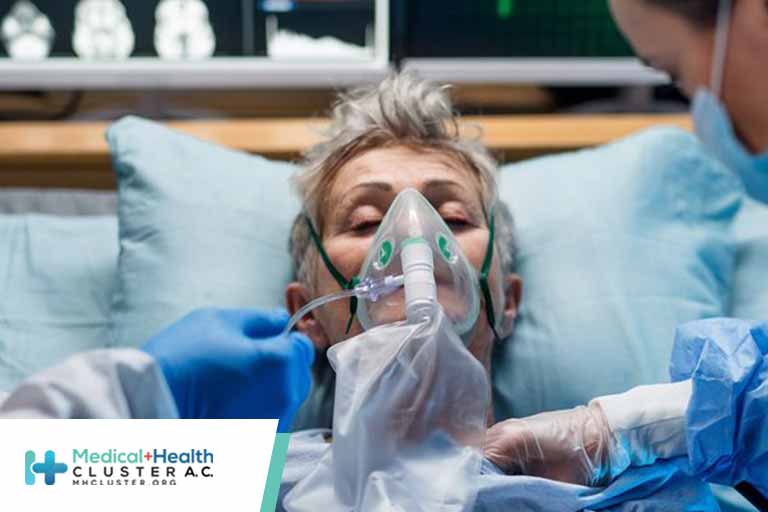
Question What are the 2-year health outcomes among patients hospitalized for COVID-19 in China? Findings In this longitudinal cohort study that included 1864 patients, the most common symptoms at 2 years after SARS-CoV-2 infection were fatigue, chest tightness, anxiety, dyspnea, and myalgia, and most symptoms resolved from 1-year to 2-year follow-up, although […]
Read More
Abstract BACKGROUND Sabizabulin is an oral, novel microtubule disruptor that has dual antiviral and anti-inflammatory activities in preclinical models. METHODS A randomized, multicenter placebo-controlled phase 3 clinical trial was conducted with hospitalized patients with moderate to severe Covid-19 who were at high risk for acute respiratory distress syndrome (ARDS) and […]
Read More
Table A. Dosing Regimens and Comments for the Drugs Recommended in Figure 2 Drug Name Dosing Regimen Comments Remdesivir Remdesivir 200 mg IV once, then remdesivir 100 mg IV once daily for 4 days or until hospital discharge Treatment may be extended for up to 10 days if there is […]
Read More
Contribution To Literature: The MICHELLE trial showed that rivaroxaban after hospitalization for COVID-19 improved clinical outcomes without increasing bleeding. Description: The goal of the trial was to evaluate rivaroxaban compared with control among patients discharged after hospitalization for coronavirus 2019 (COVID-19) infection. Study Design Randomization Parallel Participants discharged after COVID-19 […]
Read More
Compared with placebo, this Janus kinase inhibitor was associated with lower risk for death or respiratory failure in such patients. In patients hospitalized with COVID-19, excess inflammation generally underlies clinical progression. By reducing cytokine production, Janus kinase (Jak) inhibitors tamp down inflammation. Now, investigators report results of an industry-supported trial […]
Read More
Adults aged ≥65 years are at increased risk for severe outcomes from COVID-19 and were identified as a priority group to receive the first COVID-19 vaccines approved for use under an Emergency Use Authorization (EUA) in the United States (1–3). In an evaluation at 24 hospitals in 14 states,* the […]
Read More