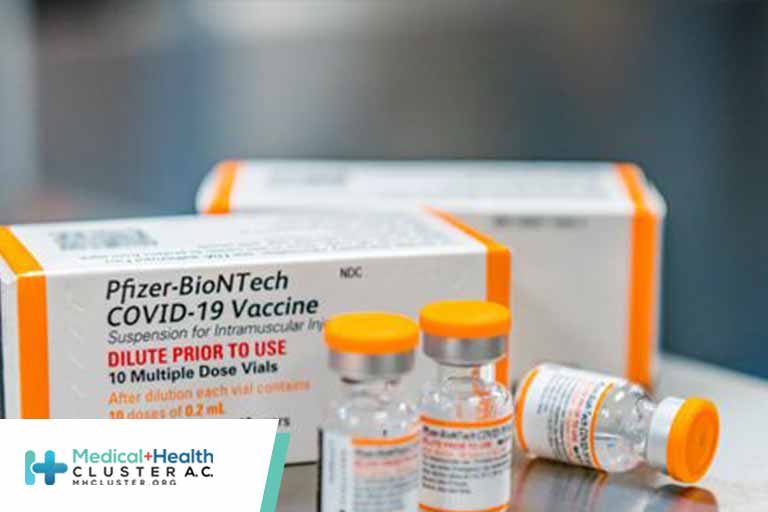
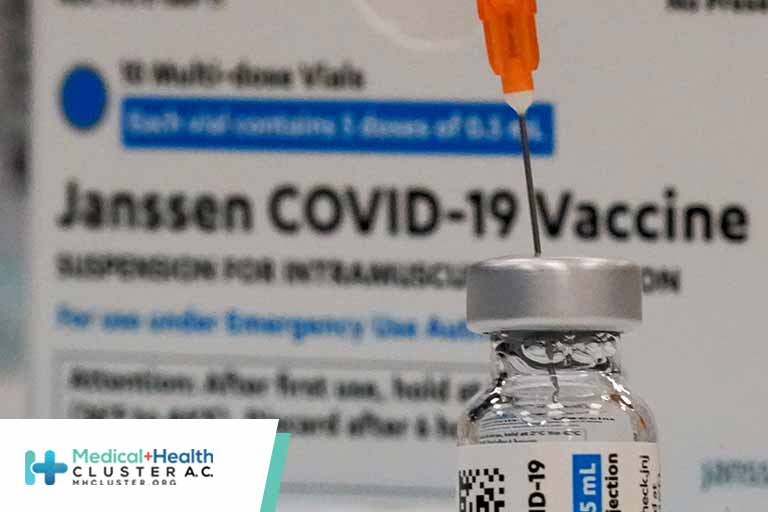
The Food and Drug Administration expanded the emergency use authorization for the Pfizer and Moderna Covid-19 vaccine boosters on Friday, signaling that the shots can be given to anyone aged 18 or older at least six months after completion of the primary vaccine series. The new policy still requires signoff […]
Read More
Pfizer plans to begin shipping millions of vials of the pediatric vaccine — with orange caps to avoid mix-ups with the purple-capped doses for everyone else — to doctors’ offices, pharmacies and other vaccination sites. WASHINGTON (AP) — The Food and Drug Administration on Friday paved the way for children […]
Read More
Washington.- Un comité asesor de la Administración de Alimentos y Fármacos (FDA, en inglés) de Estados Unidos recomendó este martes la aprobación de la vacuna contra la Covid-19 de Pfizer para menores de entre 5 y 11 años. Tras más de siete horas de reunión, los expertos de este órgano consultivo dieron luz verde a la […]
Read More
A top U.S. Food and Drug Administration official said on Friday that the agency is considering lowering the recommended age for who should receive booster shots of the Pfizer/BioNTech COVID-19 vaccine to as young as 40 years old, based on data from Israel suggesting the vaccine’s efficacy is waning. Israeli […]
Read More
The Food and Drug Administration has approved the Flucelvax quadrivalent vaccine for use in children aged 6 months and older, according to a statement from manufacturer Seqirus. “This approval officially allows all eligible Americans to receive a cell-based influenza vaccine, increasing the potential for greater vaccine effectiveness,” according to the company. The Centers for […]
Read More
Según STAT NEWS, el panel asesor de la FDA norteamericana (VRBPAC) aprobó por 19 votos a favor y ninguno en contra, autorizar la dosis de recuerdo de la vacuna Spikevax para los mayores de 65 años, los de 18 a 64 años con factores de riesgo de padecer COVID-19 grave y […]
Read More
Según CIDRAP,el comité ad hoc de la FDA norteamericana, VRBPAC, en su reunión de 15 de octubre votó por 19 a 0 la administración de una dosis de recuerdo de la vacuna de Jonhson & Johnson (Janssen) para TODOS los americanos de 18 o más años. Aunque la decisión no es […]
Read More
A US Food and Drug Administration (FDA) advisory committee on Friday voted 19-0 to authorize second doses of the Johnson & Johnson COVID-19 vaccine in an effort to boost immunity. It was the second vote in as many days to back a change to a COVID vaccine timeline. In its […]
Read More
A panel of experts that advises the FDA on vaccine decisions voted unanimously Thursday to approve booster doses of Moderna’s COVID-19 vaccine. The 19 members of the FDA’s Vaccines and Related Biological Products Advisory Committee voted to authorize a 50-milligram dose — half the dose used in the primary series […]
Read More
La farmacéuticas Pfizer y BioNtech solicitaron este jueves formalmente a la Administración de Fármacos y Alimentos (FDA) de Estados Unidos la autorización para el uso de emergencia de su vacuna contra Covid-19 en niños de 5 a 11 años. En su cuenta de Twitter, Pfizer confirma que ha hecho esta solicitud para la autorización, hasta el momento la FDA sólo autoriza el […]
Read More