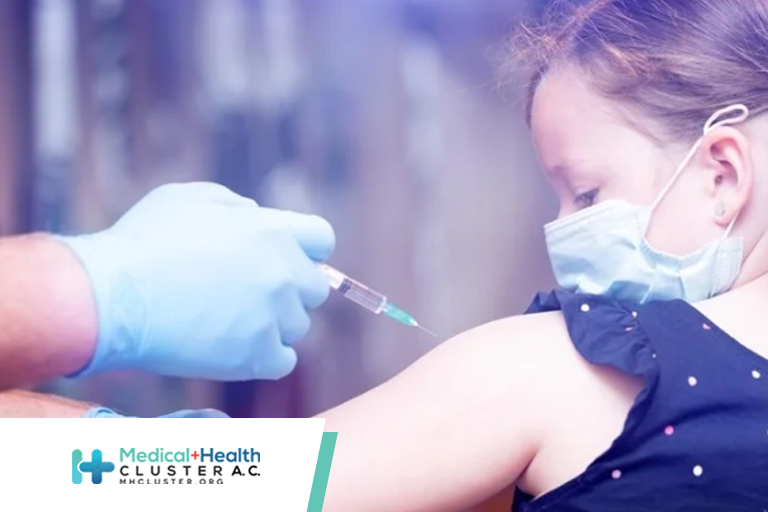
About Vaccination for Children and Teens CDC recommends COVID-19 vaccines for everyone 6 months and older and boosters for everyone 5 years and older, if eligible. Use CDC’s COVID-19 booster tool to learn if and when your child or teen can get boosters to stay up to date with their COVID-19 vaccines. […]
Read More
Abstract BACKGROUND Limited evidence is available on the real-world effectiveness of the BNT162b2 vaccine against coronavirus disease 2019 (Covid-19) and specifically against infection with the omicron variant among children 5 to 11 years of age. METHODS Using data from the largest health care organization in Israel, we identified a cohort […]
Read More
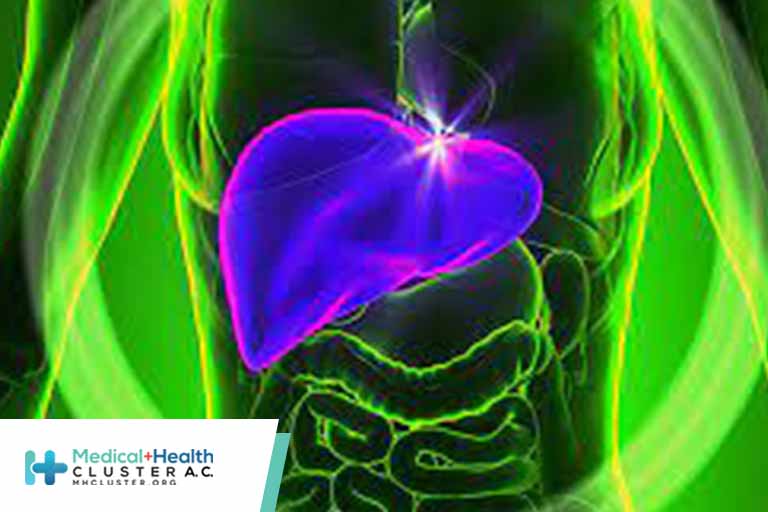
Nearly 200 cases of unexplained hepatitis have been reported in children globally, according to the European Centre for Disease Prevention and Control (ECDC). As of April 20, 111 cases have been reported in the United Kingdom, and as of April 27, 55 cases have been reported in 12 countries in the European Union: Austria, […]
Read More
Today, the U.S. Food and Drug Administration expanded the approval of the COVID-19 treatment Veklury (remdesivir) to include pediatric patients 28 days of age and older weighing at least 3 kilograms (about 7 pounds) with positive results of direct SARS-CoV-2 viral testing, who are: Hospitalized, or Not hospitalized and have […]
Read More
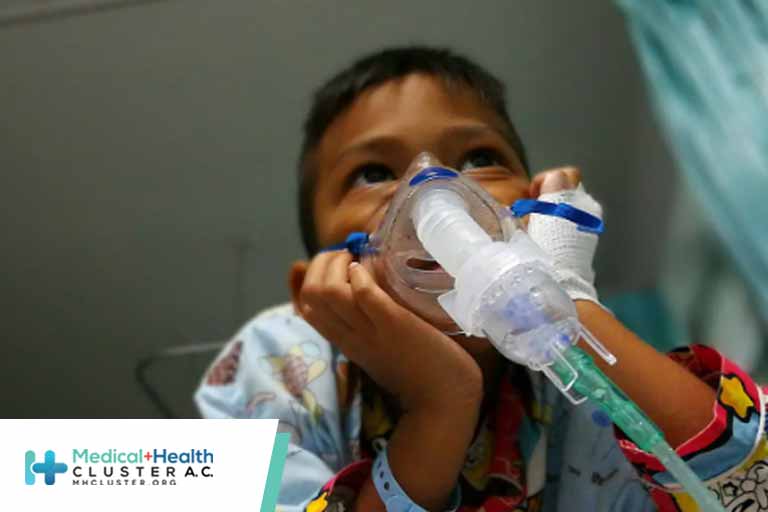
Unvaccinated children ages 5 to 11 were more than twice as likely to be hospitalized for COVID-19 during the Omicron wave, and nearly a third of hospitalized kids overall had no underlying medical conditions while about a fifth required intensive care unit (ICU) admission, researchers found. From Dec. 19, 2021 […]
Read More
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association. New cases were down to 29,000 for the week of March 18-24, a drop […]
Read More
School mask mandates helped to protect children and teachers from the coronavirus last fall, according to a new study released by the CDC. Public school districts in Arkansas with mask requirements had 23% lower rates of the coronavirus among students and staff than districts without mandates from August to October 2021 as […]
Read More
Summary What is already known about this topic? Two doses of Pfizer-BioNTech vaccine provided protection against COVID-19 in persons aged 12–17 years during Delta predominance, but data during Omicron predominance and among children aged 5–11 years are lacking. What is added by this report? Two doses protect against COVID-19–associated emergency […]
Read More
What is already known about this topic? COVID-19 can cause severe illness in children and adolescents. What is added by this report? Coinciding with increased circulation of the Omicron variant, COVID-19–associated hospitalization rates among children and adolescents aged 0–17 years increased rapidly in late December 2021, especially among children aged […]
Read More
The FDA authorized the Pfizer vaccine for children ages 5–11 in October 2021, on the basis of studies showing a robust immune response, fewer cases of COVID, and minimal side effects in several thousand children. Since that time, millions of children have been vaccinated. The latter—minimal side effects—speaks to the […]
Read More