When started within 5 days after symptom onset, molnupiravir lowered risk for post-acute death and long-COVID sequelae regardless of vaccination status or reinfection. Long COVID is a multifaceted condition with persistent post-acute and chronic sequelae. Previous reports indicated that full vaccination and antiviral treatment with nirmatrelvir/ritonavir (Paxlovid) were both associated […]
Read More
SAN DIEGO – A COVID-19 combination antiviral is the most important new drug primary care physicians have prescribed in recent years – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians. Nirmatrelvir-ritonavir was granted emergency use […]
Read More
Patients with mild to moderate COVID-19 who were treated for 5 days with VV116, an investigational oral antiviral drug that is active against SARS-CoV-2, recovered as quickly from infection as did those treated with nirmatrelvir-ritonavir (Paxlovid), according to the results of a randomized noninferiority trial published in the New England […]
Read More
La pandemia desencadenada por el SARS-CoV-2 ha cambiado desde aquel primer caso en Wuhan en 2019, actualmente contamos con eficientes vacunas que han permitido el regreso a nuestras actividades cotidianas. Asimismo, con el transcurso del tiempo se han posicionado tratamientos que están disponibles en mayor o menor medida para el […]
Read More
Pharmacists can now prescribe nirmatrelvir-ritonavir (Paxlovid) with certain limitations under a revised Emergency Use Authorization (EUA) for the COVID-19 antiviral treatment. The pills must be taken within 5 days of symptom onset, so enabling patients to obtain a prescription from pharmacists could expand access to timely treatment, Patrizia Cavazzoni, MD, director of […]
Read More
Communities most at risk of severe COVID-19 outcomes have half the rate of prescriptions compared with less vulnerable communities, according to a CDC report. More than 1 million doses of oral antiviral medications to treat individuals with SARS-CoV-2 have been prescribed since late December 2021 when the US Food and Drug […]
Read More
Summary What is already known about this topic? Lagevrio and Paxlovid are oral antiviral drugs effective at preventing hospitalization and death in patients with mild to moderate COVID-19 who are at risk for progression to severe disease. What is added by this report? During December 23, 2021–May 21, 2022, 1,076,762 […]
Read More
La ministra de Sanidad, Carolina Darias, anunció el pasado 25 de mayo en la rueda de prensa posterior al Consejo Interterritorial del Sistema Nacional de Salud (CISNS), la participación de España, junto al resto de Estados miembros de la Unión Europea, en la adquisición conjunta de Imvanex, la vacuna autorizada en julio de 2013 […]
Read More
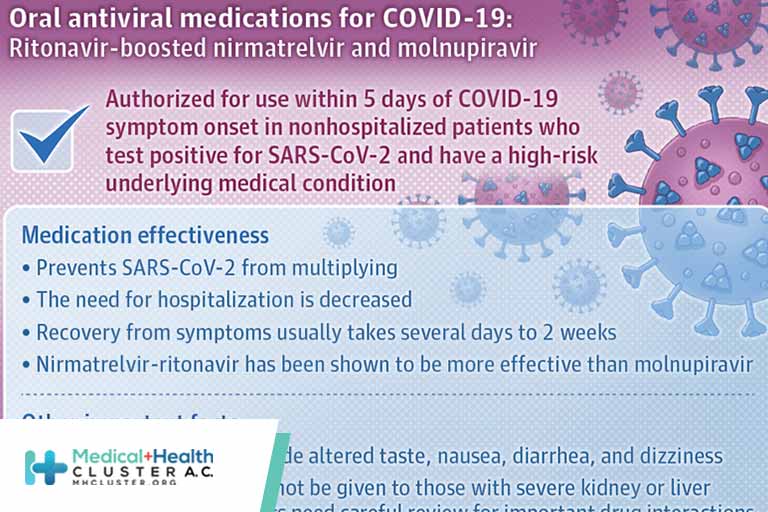
Two new oral antiviral medications are available for treatment of COVID-19. Two new antiviral medications, ritonavir-boosted nirmatrelvir (Paxlovid, ie, nirmatrelvir-ritonavir) and molnupiravir (Lagevrio), are currently available in the US under emergency use authorization. These 2 drugs are authorized for treatment of patients with mild to moderate COVID-19 who are not […]
Read More
SARS-CoV-2, the virus that causes COVID-19, has continuously mutated since it emerged in late 2019. This rapid evolution poses challenges for vaccines and treatments designed to target the virus. A booster shot is now needed to produce antibodies to fight off the Omicron variant of SARS-CoV-2. And several combinations of […]
Read More