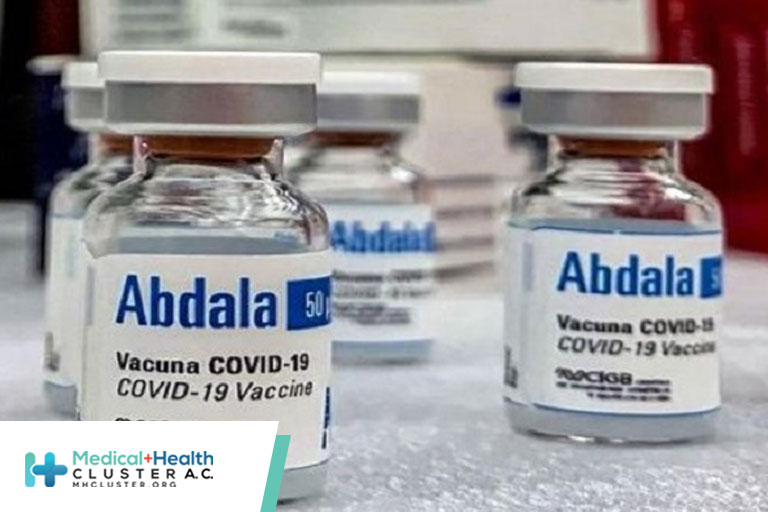
En un estudio de cohorte retrospectivo realizado en La Habana, Cuba, se analizaron las bases de datos del Ministerio de Salud Pública (12 de mayo al 31 de agosto de 2021) para evaluar la efectividad de la vacuna Abdala, demostrando que: La efectividad de la vacuna contra la enfermedad grave y […]
Read More
The WHO COVID-19 Clinical management: living guidance contains the Organization’s most up-to-date recommendations for the clinical management of people with COVID-19. Providing guidance that is comprehensive and holistic for the optimal care of COVID-19 patients throughout their entire illness is important. The latest version of this living guideline is available in pdf format (via the ‘Download’ […]
Read More
Question What are the 2-year health outcomes among patients hospitalized for COVID-19 in China? Findings In this longitudinal cohort study that included 1864 patients, the most common symptoms at 2 years after SARS-CoV-2 infection were fatigue, chest tightness, anxiety, dyspnea, and myalgia, and most symptoms resolved from 1-year to 2-year follow-up, although […]
Read More
Switching to a different COVID-19 vaccine after a first dose of the Sputnik V C1, ChAdOx1-S, or BBIBP-CorV vaccines can greatly increase immune response while maintaining safety, according to a study published in Cell Reports Medicine. The results provide information for mixing vaccine types in both primary vaccination and vaccine booster schedules, which […]
Read More
SACRAMENTO – To better align state COVID-19 guidance with the most current federal recommendations, the California Department of Public Health (CDPH) is ending COVID-19 policies that required weekly COVID-19 testing for unvaccinated individuals in high-risk workplaces and schools. Health care facilities, other congregate settings and schools will no longer be […]
Read More
El Comité de Medicamentos de Uso Humano de la Agencia Europea del Medicamento, CHMP, ha recomendado autorizar una vacuna bivariante adaptada a las subvariantes BA.4 y BA.5, que contiene, además, la cepa ancestral del SARS-CoV-2. El preparado vacunal ha sido elaborado por Pfizer/BioNTech para personas de doce o más años, con […]
Read More
IHME produced its latest COVID-19 model run on September 9. Key takeaways: Global infections peaked in August and are currently declining. We expect infections to increase in the Northern Hemisphere in October through the end of 2022, but we do not expect this to lead to major increases in severe disease and […]
Read More
The antiviral drug Paxlovid appears to reduce the risk of dying from COVID-19 by 79% and decrease hospitalizations by 73% in at-risk patients who are ages 65 and older, according to a new study published in The New England Journal of Medicine. The pill, which is a combination of the drugs nirmatrelvir and ritonavir, […]
Read More
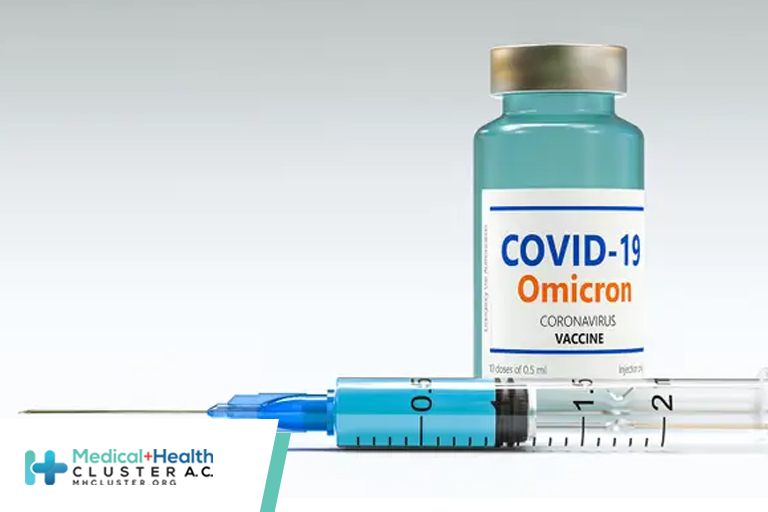
The virus that causes COVID-19 changes over time. Keep your protection up to date by getting an updated COVID-19 vaccine booster. The updated COVID-19 vaccine boosters include components of the original virus strain and the Omicron variant. This is called a bivalent COVID-19 vaccine. The updated COVID-19 vaccine boosters are […]
Read More
Expiration dates have been extended from 9 months after manufacture to 12 months for 4 lots of the COVID-19 antiviral therapy Paxlovid, which consists of copackaged nirmatrelvir tablets and ritonavir tablets. The lot numbers and original expiration dates are listed on the FDA website. The nirmatrelvir-ritonavir lots were manufactured before the FDA […]
Read More