En atención a la creciente preocupación sobre la confianza en...
Leer más
Three Effective Treatments for COVID-19 Not in Treatment Guidelines — at Least Not Yet

A few weeks ago, in a patented (and copyrighted and trademarked) Really Rapid Review™, I summarized some of the Greatest Hits from CROI 2023. The conference included new data on not just HIV, but also a grab bag of opportunistic infections, STIs, viral hepatitis — and, as has been the case since 2020, COVID-19.
You know, right in the wheelhouse of readers like you.
At least most of you. Wrote one longtime fan after that post:
Paul,
That was undoubtedly the most boring blog post you’ve ever done.
Mom
Um, certainly no one ever accused my mother of hiding her true feelings!
Risking again putting this very same reader to sleep, I bring you now more data presented at CROI — three studies highlighting promising outpatient COVID-19 treatments. The full presentations are now available on the CROI website, and I’ve linked them below:
1. Ensitrelvir. A SAR-CoV-2 protease inhibitor like nirmatrelvir, ensitrelvir at two doses was compared to placebo in a randomized trial done in people at low risk for severe outcomes — meaning younger (12–69 years old), mostly vaccinated, and lacking risk factors for severe disease.
Ensitrelvir shortened the duration of symptoms by about a day (the primary endpoint) and hastened the time to the first negative SARS-CoV-2 viral test. Perhaps most importantly for this group at low risk for hospitalization but still vulnerable to long COVID, a questionnaire targeting symptoms of long COVID conducted at 3 and 6 months showed a significant reduction in the treatment group compared to placebo. The protective effect was greater in those with more severe disease at start, the people at greatest risk of getting this complication to begin with.
Based on these results, I think these are the strongest data we have that antiviral therapy reduces the likelihood of developing long COVID. Yay to that. Note that ensitrelvir already has approval for treatment of COVID-19 in Japan.
2. Metformin. In the quest for “repurposed” drugs for COVID-19, the hits (dexamethasone, tocilizumab, baricitinib) lose badly to the misses (lopinavir/ritonavir, hydroxychloroquine, ivermectin, azithromycin, colchicine, fluvoxamine, numerous others), especially for outpatient treatment. Could metformin be the exception?
At CROI, the investigators of the COVID-OUT study presented data on their randomized clinical trial of metformin versus placebo. Treatment was significantly better in a composite outcome of emergency department visits, hospitalizations, or death; the drug also demonstrated a significant antiviral effect. Furthermore, long-term follow-up found that treated patients were less likely to receive a diagnosis of long COVID by their providers.
Add to these benefits the widespread familiarity that clinicians have with this drug, its well-established safety profile, and its extraordinarily low cost, and we might have a winner here, folks.
3. Pegylated interferon lambda. Need I say more?
I won’t pretend there aren’t issues with these three studies. Here are a few worth exploring:
 Ensitrelvir: The study evaluated two doses of the drug, 125 mg and 250 mg once daily; the lower dose appeared to be more effective, for unclear reasons. Plus, the long COVID endpoint analysis I highlighted was not protocol-specified, and hence must be considered exploratory.
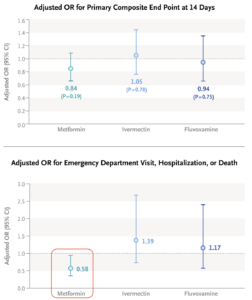
Ensitrelvir: The study evaluated two doses of the drug, 125 mg and 250 mg once daily; the lower dose appeared to be more effective, for unclear reasons. Plus, the long COVID endpoint analysis I highlighted was not protocol-specified, and hence must be considered exploratory.- Metformin: The primary endpoint of the metformin COVID-OUT study, which included home oxygenation results as part of a composite clinical endpoint, was negative. Subsequently, the investigators learned that the home oxygenation results were unreliable, which undoubtedly introduced a lot of noise into analysis of this endpoint. Note that the positive secondary endpoint for metformin (emergency room visits, hospitalization, or death) did make it onto the Research Summary (see figure — edit mine), but it’s not the message most take from the published paper.
- Pegylated interferon lambda: The TOGETHER trial enrolled patients in Brazil (mostly) and Canada; this study previously yielded favorable results with fluvoxamine. Given the subsequent negative results with fluvoxamine, should we be skeptical of any data coming from this study?
Still, there’s a lot to like here with all three treatments, especially given our limited current options now that monoclonal antibodies are gone. And importantly, the three outpatient therapies — Paxlovid, molnupiravir, intravenous remdesivir — have their own issues, some of which I’ve summarized previously.
Some might think we’re done with COVID-19, so why invest in studying further treatments? To those people, let’s face facts — this respiratory virus isn’t going anywhere, still accounts for hundreds of deaths a week in medically vulnerable populations, and causes enormous disruption in workplaces and schools. An annual bump in cases each respiratory virus season is all but a certainty given what we’ve seen the past three winters.
Source: https://blogs.jwatch.org/hiv-id-observations/index.php/three-effective-treatments-for-covid-19-not-in-treatment-guidelines-at-least-not-yet/2023/03/27/




