En atención a la creciente preocupación sobre la confianza en...
Leer más
Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape
Abstract
Where 2020 saw the development and testing of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at an unprecedented pace, the first half of 2021 has seen vaccine rollout in many countries. In this Progress article, we provide a snapshot of ongoing vaccine efficacy studies, as well as real-world data on vaccine effectiveness and the impact of virus variants of concern. Where they have been deployed in a high proportion of the adult population, the currently approved vaccines have been extremely effective in preventing COVID-19, particularly severe disease. Nonetheless, there are still significant challenges in ensuring equitable vaccine access around the globe and lessons that can be learned for controlling this pandemic and for the next pandemic.
Introduction
Fifteen months on from the administration of the first experimental COVID-19 vaccine doses to humans in March 2020, there is now a global race against the causative virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to deploy vaccines to control the pandemic1, with differing levels of vaccine rollout in different countries (Our World in Data). Despite authorization having been granted for multiple vaccines, as the ongoing global outbreaks demonstrate, the pandemic is far from over. Vaccination, in combination with non-pharmaceutical interventions, is the best way to control the pandemic. In this regard, we are fortunate that there is now a formidable toolkit of potential vaccines available: of the 322 candidate vaccines that have been proposed so far (July 2021), 99 are in clinical testing, 25 have reached phase III efficacy studies and 18 have received some form of approval for use. However, achieving global vaccine coverage remains a major hurdle; this is not just a matter of equity but is also an important part of the process to control the virus. SARS-CoV-2 continues to evolve under immune selective pressure, and while transmission levels remain high, there is an increased likelihood of vaccine escape variants evolving. In this Progress article, we cover recent developments in COVID-19 vaccines since the start of their deployment in late 2020/early 2021, including real-world data on vaccine effectiveness and the impact of viral variants.
The current vaccine landscape
Data from several phase III vaccine efficacy trials were reported at the end of 2020, leading to the approval and rollout of these vaccines. The following organizations have reported efficacy data for their vaccines as summarized in Table 1: Pfizer–BioNTech2, Moderna3, AstraZeneca–University of Oxford4, Johnson & Johnson5, Gamaleya6, Sinovac Biotech7, Sinopharm7, Novavax8 and Bharat Biotech9. As of 14 June 2021, each of these vaccines, except for the Novavax vaccine, had been approved for rollout to adults and, in some cases, to adolescents through a range of approval processes depending on the region and regulatory agency. This situation remains fluid, and importantly, the publication of the first wave of efficacy data and the first vaccine approvals were not the end of the clinical trial phase for COVID-19 vaccines. Ongoing vaccine trials test the efficacy of new vaccines in the face of an evolving virus; furthermore, they increase options for globally dispersed manufacturing of sufficient vaccine doses for global use and strengthen the data for novel vaccine platforms that could be of use in future pandemics. As this is a rapidly moving space, the best way to stay abreast of the vaccine trials is through live documents, such as the COVID-19 vaccine tracker from the London School of Hygiene and Tropical Medicine.
Within this larger list of ongoing studies, a smaller number of vaccines are in phase III clinical trials that are yet to release data: Inovio (DNA, ClinicalTrials.gov identifier NCT04336410), AnGes (DNA, NCT04655625), ReiThera (gorilla adenovirus, NCT04791423), the Chinese Academy of Medical Sciences (inactivated virus, NCT04412538), the Research Institute for Biological Safety Problems, Kazakhstan (QazCovid; inactivated virus, NCT04691908), Shifa (inactivated virus, NCT04526990), the Center for Genetic Engineering and Biotechnology, Cuba (CIGB-66; peptide, RPCEC00000345), Clover (peptide, NCT04672395), COVAXX (peptide, NCT04545749), the Finlay Institute, Cuba (peptide, RPCEC00000332), Sanofi–GlaxoSmithKline (adjuvanted protein, NCT04904549), VECTOR (peptide, NCT04780035) and Medicago (plant-derived virus-like particle, NCT04636697). We anticipate that some of the candidate vaccines currently in phase III trials will become available for wider use in quarters 3 and 4 of 2021, which will be important in stemming further waves of the pandemic and increasing vaccination rates in lower-income countries.
However, it should also be noted that some fairly high-profile vaccine programmes may not enter phase III efficacy studies. In collaboration with IAVI, Merck developed the viral-vectored COVID-19 vaccine V590 (ref.10). Merck also acquired a second COVID-19 vaccine through the buyout of Themis, the company responsible for producing the V591 (live viral vector) vaccine. However, the development of both V590 and V591 has been discontinued, owing to their poor immunogenicity11. The University of Queensland and the biotechnology company CSL also discontinued development of their protein subunit vaccine candidate (UQ-CSL v451). Phase I trials showed that the vaccine was well tolerated and elicited a robust immune response. However, trial participants also developed antibodies to the ‘molecular clamp’ used to stabilize the SARS-CoV-2 spike (S) protein12, which comprised S protein fragments from HIV-1 (ref.13). Antibodies to the molecular clamp were shown to interfere with HIV-1 tests, causing false-positive results, which halted the development of the vaccine12,13. Whereas the mRNA vaccines from Pfizer–BioNTech (BNT162b2) and Moderna (mRNA-1273) have shown high levels of efficacy, a third mRNA candidate vaccine, from CureVac (CVnCoV), demonstrated only 47% efficacy in interim analysis, which did not meet the trial success criteria14. It is not yet clear why CVnCoV was less protective than either BNT162b2 or mRNA-1273, but it is of note that unlike the mRNA used in the Pfizer–BioNTech vaccine or the Moderna vaccine, the CureVac mRNA does not contain the modified nucleoside pseudouridine instead of uridine, which may alter the way the vaccine itself is sensed15. The Sanofi–GlaxoSmithKline adjuvanted protein vaccine experienced a setback when the initial trial used too low a dose of antigen16, but following reformulation, this programme is ongoing with a phase III trial.
The approved vaccines use a range of different platforms (mRNA, viral vector, protein/peptide and inactivated virus). Comparisons of the relationship between efficacy and neutralizing and binding antibody titres in vitro across various vaccine platforms have been conducted17,18. These data suggest higher antibody responses to the mRNA vaccines and to the Novavax protein subunit vaccine than to the viral-vectored and inactivated virus vaccines. Dissecting why different vaccine platforms induce a different quality and/or quantity of immune response will be crucial to develop successful vaccine approaches for future pandemics. Efficacies ranging from 60% to 94% have been reported for the various vaccines, but owing to differences in trial design, end point measured, trial location, population studied and prevalence of SARS-CoV-2 variants at the time of the trial, it is not possible to make head-to-head comparisons between the different vaccine platforms. Although differences in how the clinical trials were set up make comparison between vaccines harder, having multiple approaches to reach the critical end goal of an effective vaccine is pragmatically more important than delaying studies to enable direct comparisons to be made, which could delay vaccine rollout and thereby contribute to increased deaths. The only valid way to compare vaccines directly is in head-to-head efficacy trials, which are unlikely to be undertaken owing to the number of trial participants required, the pre-existing efficacy data, the current level of vaccine rollout and levels of pre-existing immunity in the population. One approach to avoid difficulties of comparison between vaccine platforms for future pandemics would be to have pre-prepared (and standardized) trial protocols that could be adopted rapidly by multiple teams. In the absence of direct comparison, the next best thing is to compare the approved vaccines for effectiveness in the same population at the same time (see the next section). An additional challenge for assessing both the efficacy and the effectiveness of vaccines is the lack of published data, with many results having been made available through press releases rather than peer-reviewed articles.
Vaccine effectiveness
The efficacies for COVID-19 vaccines shown in Table 1 were calculated from clinical trials based on defined end points (which can differ between trials, even in terms of the definition of symptomatic disease). These values are important for the approval of vaccines, but they do not necessarily reflect the impact of vaccination in the real world, especially when clinical trials for vaccines have enrolled mostly younger, healthy adults compared to those most at risk of severe disease, and these trials occurred in the absence of some of the more recently reported SARS-CoV-2 variants. It is therefore important to understand the extent and duration of protection against infection or disease in all age groups and populations; in the case of COVID-19, this is particularly important given the higher risk of severe disease in older individuals (70 years of age and older). Vaccine effectiveness, which differs from vaccine efficacy, is the reduced risk of infection or disease among vaccinated individuals. This can be impacted by population-dependent effects of the vaccine as well as vaccination schedules and handling/administration of vaccines (Fig. 1).
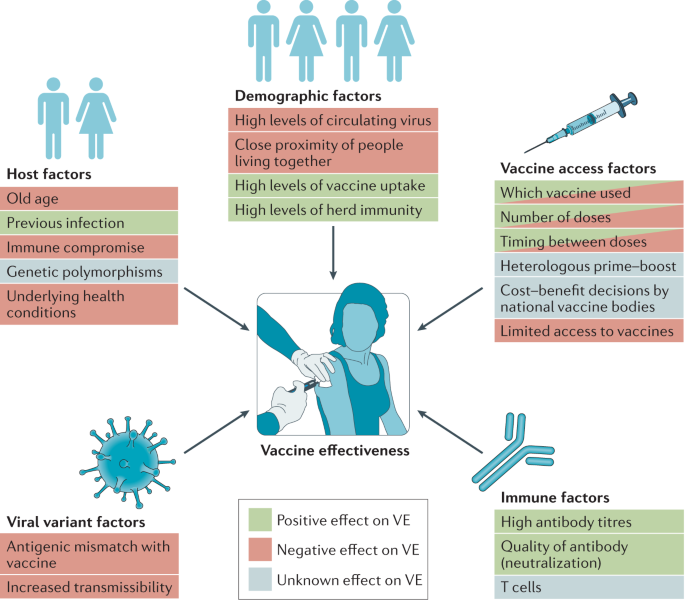
Multiple factors can increase or decrease vaccine effectiveness (VE) at both the individual level and the population level.
In the UK, vaccination with BNT162b2 (Pfizer–BioNTech) or AZD1222 (AstraZeneca–University of Oxford) started on 3 December 2020. Since April 2021, the mRNA-1273 (Moderna) vaccine has also been available in the UK, but owing to the shorter time that this has been part of the vaccination schedule, there are as yet no vaccine effectiveness data for mRNA-1273 from the UK. Public Health England reported on the early impact and effectiveness of COVID-19 vaccination with BNT162b2 or AZD1222 in England in March 2021: vaccine effectiveness against death and hospitalization for either vaccine was estimated at 81% and 80%, respectively19. In a study of the effectiveness of BNT162b2 or AZD1222 against symptoms in 156,930 adults between December 2020 and February 2021, vaccine effectiveness was 70% in participants aged 80 years or older after the first vaccine dose, increasing to 89% 14 days after the second dose20. In the same study, for participants aged 70 years or older, vaccine effectiveness against symptoms was 61% for BNT162b2 and 60% for AZD1222 28–34 days after first-dose vaccination. However, a potential limitation of these early estimates of vaccine effectiveness is that the controls were defined differently and thus odds ratios may be skewed. The studies were either test-negative designs (in which the control group is individuals within the study who have COVID-19-like symptoms but test negative for SARS-CoV-2) or based on a screening method (in which vaccination coverage in SARS-CoV-2-positive individuals is compared with the vaccination coverage in the general population from which the cases are derived). Using vaccination coverage as the control can produce more reliable information on vaccine effectiveness, such as the method used in the SIREN (SARS-CoV-2 Immunity and Reinfection Evaluation) study21.
In the UK, a decision was made on 30 December 2020 to delay the second vaccine dose to 12 weeks after the first dose, regardless of the vaccine administered. This was a pragmatic decision aiming to increase the number of individuals (particularly in high-risk groups) who would be afforded some immune protection from a first dose. The 12-week interval differed from the regime used in the efficacy trials for BNT162b2 and mRNA-1273, which gave doses at 3-week and 4-week intervals, respectively. There were concerns that immunity might wane rapidly after a single dose, that in more vulnerable individuals the response to a single dose might be insufficient and that lower levels of immunity might in some way accelerate the evolution of viral variants that could escape the immune response. These questions have yet to be fully answered and will continue to be important considerations especially as the UK continues to ease non-pharmaceutical interventions22. Unsurprisingly, both antibody responses23 and vaccine effectiveness24 are lower after a single dose of either BNT162b2 or mRNA-1273 than after two doses. Antibody levels following BNT162b2 vaccination do wane within 12 weeks following one dose23. There is, however, protection from one vaccine dose, with vaccine effectiveness after a single dose of BNT162b2 of 91% and after a single dose of AZD1222 of 88%24. One advantage of delaying vaccination is that antibody responses to the second dose might be greater; the antibody response to BNT162b2 is greater in individuals aged 80 years or older when the vaccination gap is increased from 3 to 12 weeks25. Ultimately, the length of the dose interval comes down to a decision by national vaccine committees as to whether it is better to get a lower level of immune protection in a greater number of people or more protection in fewer people. This risk–benefit profile will change over time, especially once greater coverage has been achieved in the elderly, more at risk groups, but also in response to the emergence of new viral variants (Table 2). In June 2021, individuals aged 40 years or older in the UK were invited to schedule their second vaccination after 8 weeks rather than 12 weeks as cases of the Delta (B.1.617.2) variant increased. Another important factor that must be included in any risk–benefit calculations is the potential serious adverse effects associated with the vaccines (Box 1).
In Israel, which was one of the first countries to vaccinate a high proportion of the adult population and hence have a more comprehensive dataset regarding vaccine effectiveness, the national immunization programme started on 20 December 2020 using BNT162b2. Individuals at high risk of severe COVID-19 were prioritized before the vaccination programme was quickly expanded to include all individuals aged 16 years or older, with a view to reducing SARS-CoV-2 transmission. Initial data indicated a single-dose vaccine effectiveness against new infections of 51% 13–24 days after immunization26. Viral load in infected individuals, as measured by reverse transcription–PCR for the SARS-CoV-2 N and S genes, was significantly lower in those who had been vaccinated than in unvaccinated individuals 12 days after vaccination27. Another study estimated vaccine effectiveness for documented SARS-CoV-2 infection at 46% after the first dose of BNT162b2 and 92% after the second dose28, given at 3 weeks. Two doses of BNT162b2 were shown to reduce symptomatic cases of COVID-19 by 94% in a dataset of 1.2 million people28.
In the USA, there was an 82% reduction of reverse transcription–PCR-positive new cases in vaccinated health-care workers versus unvaccinated health-care workers 14 days after the first dose of either BNT162b2 or mRNA-1273 (ref.29). In another study, conducted between December 2020 and March 2021, vaccine effectiveness against infection after two doses of BNT162b2 or mRNA-1273 among health-care personnel was 90%30. In a multistate analysis of adults aged 65 years or older receiving BNT162b2 or mRNA-1273, vaccine effectiveness against hospitalization for COVID-19 was 95% after two doses and 64% after one dose31. Vaccine effectiveness against infection in a large cohort of 49,220 US health-care workers with a median age of 41 years exceeded 96% after two doses of BNT162b2 or mRNA-1273 (ref.32).
The efficacy data for the vaccines produced by Sinovac Biotech (CoronaVac) and Sinopharm (BBIBP-CorV) have not yet been published in a peer-reviewed journal, and similarly the vaccine effectiveness data for these vaccines are not available, even though the vaccines have Emergency Use Listing by the World Health Organization (WHO) and have been widely administered, particularly in low-income countries. Of concern, some countries with relatively high vaccination rates continue to have high levels of infection; for example, Chile, which has immunized 50% of the population (87% of whom received CoronaVac), experienced 70,000 new cases a day in June 2021. A study conducted between February and May 2021 indicated that vaccine effectiveness was 66% for the prevention of laboratory-confirmed COVID-19 (ref.33). More openly available data are urgently required on the efficacy and effectiveness of these vaccines.
Taken together, the results show that where vaccine effectiveness data have been published, the findings are broadly consistent with efficacy data determined from clinical trials. Given the range of immune responses that are generated by different vaccine platforms, it will be essential to further understand what immune mechanism provides protection in each of these cases.
Correlates of protection
The mechanism of protection of COVID-19 vaccines is still not completely clear34. A measurable correlate of protection that reliably predicts protection against COVID-19 after vaccination or natural infection has not yet been defined. Better understanding of the mechanisms of protection will be important for comparing different vaccines and to accelerate the rollout of vaccines against future viral variants. Vaccination induces both humoral and cellular responses, but it is widely thought that vaccine-induced neutralizing antibodies to the receptor-binding domain of the SARS-CoV-2 S protein are a plausible mechanism of protection. Neutralizing antibodies to the S protein provide near-complete protection against rechallenge in animal studies35. Recent modelling studies have suggested that neutralizing antibodies are highly predictive of protection against infection or severe disease36, with a second modelling study suggesting a tight correlation between neutralizing antibody levels and reported efficacy across several COVID-19 vaccine trials18, and a third study derived from efficacy trials indicating that both binding and neutralizing antibody titres correlate with protection37.
Antibody-mediated protection reflects experience with influenza vaccines, and an assay measuring antibody function (equivalent to the haemagglutination inhibition assay used for influenza antibodies) will probably be the best tool to predict protection, as it is the easiest to standardize and distribute. However, the exact quality and quantity of vaccine-generated antigen-specific functional antibodies required to protect against SARS-CoV-2 reinfection in humans are not yet known. A combination of studies will be necessary to determine the best correlate of protection for SARS-CoV-2. One approach is to establish prospective cohorts of previously infected or vaccinated individuals and monitor these cohorts for subsequent SARS-CoV-2 infection; the banked material can then give an indication of levels of immunity that are not protective. This can be done in parallel with vaccine efficacy trials, comparing immune responses to vaccine in participants who develop COVID-19 with those who do not, although this requires there to be a relatively high vaccine failure rate. These studies can be combined with human challenge studies38, which allow direct testing of infection in individuals with known levels of pre-existing immunity. In early 2021, pilot SARS-CoV-2 human infection challenge studies were established at Imperial College London, UK, and the University of Oxford, UK, which will hopefully contribute to a better understanding of immune correlates. Neutralizing antibodies most likely have a protective role, but other mechanisms of antibody-mediated protection may also contribute, for example through the constant domain of the antibody such as antibody-dependent cell-mediated cytotoxicity, as well as cell-mediated immunity. One consideration regarding neutralizing antibodies is that they may be more sensitive to escape by viral variants as they target a focused region of the S protein.
Effects of viral variants
Early in the COVID-19 pandemic, the number of ‘mutant’ variant viruses was low owing to the small number of people infected with the virus (and hence fewer opportunities for escape mutants to emerge). Since then, the huge number of infections, including prolonged infection in immunocompromised individuals, has led to the evolution of multiple SARS-CoV-2 variants. Understanding the impact of these variants on the success of public health and vaccination programmes is of paramount importance39. Although new variants continue to emerge, the most exhaustive information at present is on four variants of concern (VOCs); a VOC is defined by the WHO as a virus with mutations compared with the reference genome found in multiple clusters with either increased transmission or virulence or decreased impact of vaccines and therapeutics40. The VOCs were recently renamed by the WHO as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2). These strains predominantly have changes in the S gene compared with the reference (Wuhan) strain (Table 2). The high frequency of mutations in the S protein has caused global concern because these mutations could alter interactions with the host receptor ACE2, thereby changing the infection rate, or could modify the potency of neutralizing antibodies, thereby compromising vaccine efficacy. Here we discuss what we know so far about vaccine efficacy against these VOCs (Table 2).
Alpha (B.1.1.7) variant
So far, only low or no significant impact on vaccine effectiveness has been reported as a result of the B.1.1.7 variant. Only slight effects of some of the mutations present in the B.1.1.7 variant have been seen in virus neutralization studies using sera from BNT162b2-vaccinated individuals41. However, the modified virus used in this study lacked the full repertoire of S protein mutations from B.1.1.7 (ref.41). A significant reduction in neutralization titres in serum from BNT162b2-immunized individuals was observed with a pseudovirus that contains the complete set of B.1.1.7 mutations42. However, there was no significant impact on the neutralizing capacity of sera from humans or non-human primates who received mRNA-1273 against the B.1.1.7 variant43. Live virus neutralization activity of sera from AZD1222-immunized individuals was ninefold lower against the B.1.1.7 variant than against a canonical non-B.1.1.7 lineage44. Serum from individuals immunized with Ad26.COV2-S (Johnson & Johnson) was able to neutralize the B.1.1.7 variant in vitro, although less efficiently than the reference strain45.
Although these in vitro studies have limitations regarding methodology and sample size or by considering only the humoral arm of the immune response, when taken together, they indicate that the efficacy of the vaccines should be similar or only slightly lower against the B.1.1.7 variant. This is supported by clinical studies. Novavax reports that its COVID-19 vaccine NVX-CoV2373, which includes the S protein from the SARS-CoV-2 Wuhan reference strain, has shown 86% efficacy against the B.1.1.7 variant (96% efficacy against the original strain) in a phase III clinical trial involving 15,000 participants aged between 18 and 84 years in the UK46. The effectiveness of AZD1222 against nucleic acid amplification test-positive infection with B.1.1.7 was 70%, whereas for non-B.1.1.7 lineages, vaccine effectiveness was 77%44.
Beta (B.1.351) variant
The K417N and E484K mutations in B.1.351 significantly affect the neutralization of this variant by both monoclonal antibodies and immune sera derived from convalescent patients47,48. B.1.351 is 6.5-fold more resistant than wild-type pseudovirus to neutralization by sera from individuals vaccinated with BNT162b2 (ref.49). A significant reduction in the neutralization of B.1.351 by sera from humans or non-human primates vaccinated with mRNA-1273 was also observed43. Antibody responses and memory B cells from recipients of mRNA-1273 or BNT162b2 showed decreased activity against SARS-CoV-2 variants containing E484K and N501Y mutations or the triple combination of K417N, E484K and N501Y (as found in B.1.351)50.
Whether this effect on antibody-mediated neutralization impacts vaccine-mediated protection is not fully known. The earliest indications come from clinical trials that have been conducted in populations where the new variants are circulating widely, although efficacy against viral variants is inferred mainly indirectly from VOC prevalence at the time of the trial. Interim efficacy results have been reported from two randomized placebo-controlled clinical trials conducted by Novavax and Johnson & Johnson in South Africa. NVX-CoV2373 showed an efficacy of 49% against the B.1.351 variant in the prevention of mild, moderate and severe COVID-19 (ref.46), with efficacy increasing to 60% when HIV-positive individuals are excluded from the analysis46. In the ENSEMBLE trial by Johnson & Johnson, the single-shot Ad26.COV2-S vaccine had 72% efficacy against PCR-confirmed infection in the USA, but these values were reduced to 66% efficacy in Latin America and 57% efficacy in South Africa45, which may reflect a higher prevalence of B.1.351 in South Africa than in the USA. Ad26.COV2-S remained 85% effective overall in preventing severe COVID-19 across all regions51. The trial of AZD1222 in South Africa did not demonstrate protection against mild to moderate B.1.351-induced COVID-19; it is yet to be determined whether this vaccine offers protection against severe disease and death52.
Gamma (P.1) variant
Considering the high number of S protein mutations that the P.1 variant has accumulated, it is reasonable to conclude that it will be equally or even more resistant than the B.1.351 variant to antibody-mediated protection. Laboratory serum neutralization assays using a pseudovirus have shown that the neutralizing activity of BNT162b2-elicited antibodies to B.1.1.7-spike virus and P.1-spike virus is approximately equivalent53. A trial in Brazil using the CoronaVac (Sinovac Biotech) vaccine showed an efficacy of 50% against symptomatic infection (just above the approval threshold for emergency use) in 12,508 volunteers, all of whom were health-care professionals in regular direct contact with SARS-CoV-2 (ref.54); however, infection with the P.1 variant was not confirmed but is assumed on the basis of the prevalence of circulating strains.
Delta (B.1.617.2) variant
A significant decrease in neutralizing antibody titre has been seen for B.1.617.2 compared with B.1.1.7 using sera from individuals immunized with BNT162b2. Vaccine effectiveness of 88% or 67% against symptomatic disease following infection with B.1.617.2 has been observed in England after two doses of BNT162b2 or AZD1222, respectively55. However, it was not possible to estimate the vaccine effectiveness against severe disease because at the time of the study (April to June 2021) there were few severe cases. Although the AZD1222 and BNT162b2 vaccines were both effective at reducing the risk of infection and hospitalization owing to B.1.617.2 in Scotland, the level of protection was not as high as against B.1.1.7 (ref.56).
In summary, across all the VOCs, a reduction of in vitro serum neutralization activity has been observed in highly sensitive assays, and there has been evidence of infection with VOCs in vaccinated populations, but the severity of disease is nevertheless much reduced, indicating that the vaccines are still highly effective. Prevention of severe disease, which could overwhelm hospitals and lead to death, is the most important goal of vaccination. Undoubtedly more VOCs will arise, and the impact of these on vaccine effectiveness is hard to predict; importantly at a time when total global vaccine coverage is low, increased transmission rather than vaccine escape is probably the main selective pressure.
To boost or not to boost?
To counteract the impact of viral variants, one suggestion is to develop new vaccines that more closely reflect the circulating viruses. For example, Moderna has developed a novel vaccine targeting the B.1.1.7 VOC, which has been tested in preclinical trials57 and is now in clinical trials (NCT04785144). However, it is not clear how beneficial such vaccines designed specifically to target new variants will be. The main consideration will be how far the circulating viruses in autumn 2021 (when booster vaccination has been proposed in some countries) will have drifted antigenically from the original reference sequence of the SARS-CoV-2 S protein used for the first-generation vaccines. Although studies relating to the VOCs have shown reduced neutralization in vitro, there has been no significant reported impact on vaccine effectiveness, which suggests that the viral mutations predominantly increase transmissibility, but not necessarily immune escape. As the current vaccines still offer good protection against severe disease, there may be limited return on a new variant booster. Indeed, there may actually be negative unintended consequences. The first is that producing a new booster vaccine for the countries with sufficient income to afford substantial coverage with the first-generation vaccines may reduce manufacturing capacity for doses for lower-income countries. Second, boosting with a similar antigen may boost the antibody response to the original strain rather than prime for antibodies specific to the new strain58. This idea of ‘original antigenic sin’ refers to the boosting of responses to previously seen epitopes to the detriment of responses to new epitopes, particularly when they are closely related. Such a phenomenon has been observed for influenza, with individuals who were recently immunized with seasonal influenza vaccine producing lower antibody responses to 2009 pandemic influenza than previously unimmunized individuals59. Furthermore, the variants may be drifting apart, and so priming with a variant might narrow rather than broaden protection. It is our opinion that engineering novel booster vaccines should not be a priority at this time.
Whereas engineering new variant vaccines may not be beneficial, an alternative strategy is to boost immunity with a third dose of vaccine targeting the initial reference strain. One important consideration is how rapidly and to what extent immunity wanes after vaccination. There were initially concerns that immunity would wane rapidly, but recent studies have observed sustained B cell-mediated immunity 12 months after initial infection60,61, although other components of the immune response, such as T cell responses, may wane faster62. However, there may still be benefits to an additional booster dose of vaccine. Whether this should be with the same vaccine as used for the initial course or with a heterologous platform is not yet known, and studies are ongoing to explore the impact of heterologous prime–boost vaccination on immunity. There are also important considerations about equity — if individuals in lower-income countries have not yet received any vaccine doses, is it fair to offer booster vaccination to individuals in higher-income countries?
Deployment issues
The challenge to increase vaccine coverage is twofold: overcoming vaccine hesitancy in the countries that do have access to vaccines but are being slow to distribute them and getting vaccine stock to countries in need. In some countries, vaccine hesitancy is clearly a challenge, with variable rates of vaccine uptake both between countries and within different populations in the same country63. Hesitancy (or no hesitancy) regarding vaccination is changing almost as quickly as the vaccine trial landscape, and live resources such as the Vaccine Confidence Project provide updated coverage.
Compared with vaccine hesitancy, global vaccine equity and access is the far bigger hurdle. There is no quick fix to increase global vaccine supply, which involves issues of reagent supply, qualified manufacturing staff, manufacturing capacity, regulatory hurdles and distribution, even without the larger context of vaccine nationalism. One problem that has been discussed prominently is intellectual property. In early May 2021, the USA proclaimed support for waiving COVID-19 vaccine patents64. Unfortunately, waiving intellectual property rights is unlikely to overcome any of the problems described. Should COVID-19 vaccine patents be lifted, there are still major hurdles regarding technology transfer, training and good manufacturing practice regulations required to produce vaccines with sufficient quality, while regulators would require safety and efficacy trials to be completed for unique products. Because vaccines are complex biological products, licensure and partnership agreements may be a more effective route to increase supply, so that technical know-how and cell seed stocks can be shared; for example, as AstraZeneca has done with the Serum Institute of India. The most important step now is to send vaccines to the countries that need them the most, prioritizing the most at risk on a global level rather than a national level. One of the key lessons from this pandemic is that a more globally distributed vaccine manufacturing infrastructure is required.
Conclusions
There are still many questions posed by the COVID-19 vaccine effort that need to be addressed, both in the context of this pandemic and for future pandemics (Box 2). The development and deployment of vaccines for COVID-19 is a remarkable science success story: within 16 months of the first vaccine trials, 2.8 billion vaccine doses have been administered. A crucial question is whether this success can be replicated in future pandemics. Pre-existing investment in vaccine platform technologies was crucial for the speed of the response: three of the most rapidly approved vaccines (BNT162b2, mRNA-1273 and AZD1222) use novel technologies. mRNA vaccines had never been tested in an efficacy trial for infectious disease before 2020, and viral vectors had been successfully deployed only against Ebola virus. Interestingly and despite concerns about vaccine-induced disease enhancement, the other vaccine platform to be rapidly developed was a much older technology — viral inactivation — as used in CoronaVac and BBIBP-CorV. This platform-specific approach was important in accelerating the development of SARS-CoV-2 vaccines; whether the same platforms will work for all pathogens is not known, so maintaining a broad portfolio of platforms will be necessary. Having multiple vaccine platforms available also led to more vaccines in the pipeline and therefore a greater manufacturing capacity. Another important factor was the ability to transfer understanding from similar pathogens: AZD1222 was developed from a Middle East respiratory syndrome coronavirus programme, and the protein engineering approach used to stabilize the S protein was derived from understanding of the respiratory syncytial virus fusion glycoprotein and the difference between prefusion and postfusion states, which was made possible only through long-term investment. Faster responses came from smaller biotechnology companies and academic institutions (with support from larger companies to scale up); whether this reflects a nimbler response or a different risk profile is unclear. All of these considerations lead to a conclusion that a broad research base is the best approach to prepare for an unknown future pathogen. As a final note, most of the COVID-19 vaccine doses have been administered in high-income or middle-income countries; as of June 2021, only 0.9% of people in low-income countries had received at least one dose (Our World in Data). In parallel with ongoing investment in research, investment in manufacturing capacity, training and the ability to deliver vaccines globally is crucial to build on the incredible successes of the past 18 months.
https://www.nature.com/articles/s41577-021-00592-1
Créditos: Comité científico Covid




