DISEASE SURVEILLANCE
The Overcoming COVID-19 surveillance registry was funded by the Centers for Disease Control and Prevention (CDC) to conduct surveillance of Covid-19–related severe complications, including MIS-C as defined according to CDC criteria,18 in children and adolescents hospitalized in the United States and to collect detailed data. Patients with MIS-C were identified at each site by intensive care unit (ICU) and subspecialty clinicians and through mandated public health reporting. Trained staff at participating facilities abstracted medical records onto a standard form and entered data into a Web-based secure electronic database (Research Electronic Data Capture [REDCap], Vanderbilt University). Data collected included the demographic characteristics of the patients, underlying medical conditions, signs and symptoms at presentation, clinical course, laboratory test results, diagnostic studies, treatments, complications, and outcomes. The surveillance protocol, which is available with the full text of this article at NEJM.org, was approved by the central institutional review board at Boston Children’s Hospital, with a waiver of informed consent. It was also reviewed by the CDC, and all activities were conducted in accordance with applicable federal law and CDC policy. The last two authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
CASE DEFINITION
Cases were adjudicated by the principal investigators at each site and at the central coordinating center. The case definition of MIS-C included six criteria18: serious illness leading to hospitalization, an age of less than 21 years, fever (body temperature, >38.0°C) or report of subjective fever lasting at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement (i.e., involving at least two organ systems), and laboratory-confirmed SARS-CoV-2 infection (positive SARS-CoV-2 real-time reverse-transcriptase–polymerase-chain-reaction [RT-PCR] or antibody test during hospitalization) or an epidemiologic link to a person with suspected or confirmed Covid-19 within 4 weeks before the onset of MIS-C symptoms. Owing to a lack of SARS-CoV-2 testing in the spring of 2020, cases reported between March 15 and May 31 as suspected or confirmed MIS-C cases that were epidemiologically linked to a Covid-19 exposure were included without the requirement of a positive SARS-CoV-2 test; positive testing was required after May 31.
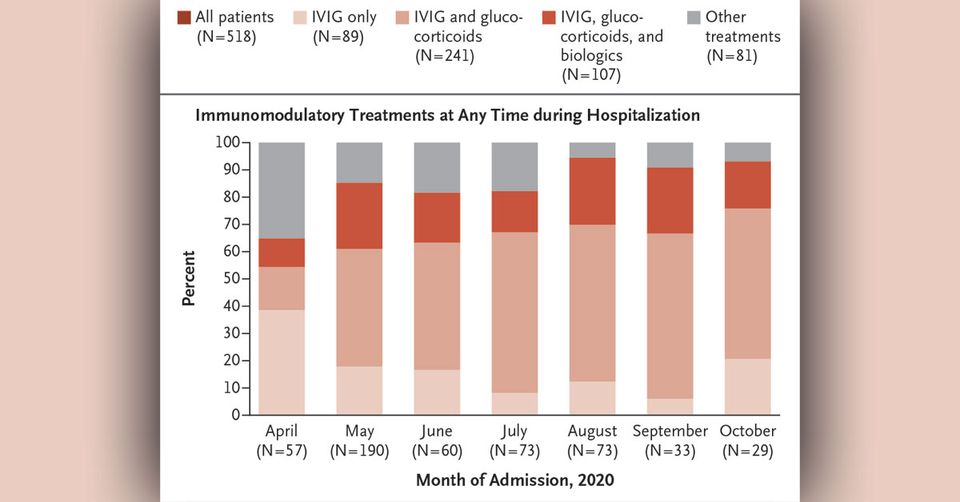
IMMUNOMODULATORY TREATMENTS
Two approaches were used to categorize patients with MIS-C. First, we categorized patients according to the immunomodulatory treatments they received at any time during their hospital course: IVIG only; IVIG and glucocorticoids; IVIG, glucocorticoids, and a biologic agent; or other treatments (glucocorticoids only, a biologic only, glucocorticoids and a biologic, or IVIG and a biologic). Glucocorticoids included methylprednisolone, prednisolone, and dexamethasone. Biologics included an interleukin-1–receptor antagonist (anakinra), tumor necrosis factor α inhibitors (infliximab and etanercept), and an interleukin-6–receptor antagonist (tocilizumab).
Second, we classified patients according to which of two commonly used19 treatments they received on day 0, which was termed their “initial treatment”: IVIG plus glucocorticoids or IVIG alone. Day 0 was defined as the first day a patient received any immunomodulatory treatment (which was not necessarily the first day of hospitalization). Days 1 and 2 were the first and second calendar days after day 0. Available data included the calendar day for all the treatments but not the calendar time. Glucocorticoids (in patients not already receiving glucocorticoids on day 0), biologics, and second doses of IVIG administered on or after day 1 were considered “adjunctive” treatment. Patients who received a biologic on day 0 were excluded because of small sample size and the use of multiple types of biologics with varying cytokine targets.
OUTCOMES
We report the following outcomes for patients who received any immunomodulatory treatment during hospitalization: admission to the ICU, receipt of supplemental oxygen, receipt of mechanical ventilatory support, use of vasopressor agents, receipt of extracorporeal membrane oxygenation, hospital length of stay, coronary-artery aneurysms, and death. Coronary-artery aneurysms were defined as a z score of at least 2.5 for the left anterior descending coronary artery or the right coronary artery (or both) on echocardiography.7
To assess the potential effectiveness of initial treatment with IVIG plus glucocorticoids, as compared with IVIG alone, we prespecified a primary outcome of cardiovascular dysfunction on or after day 2 through the time of discharge, which was based on a composite of left ventricular dysfunction (as defined by a left ventricular ejection fraction [LVEF] of <55% on echocardiography) or shock that resulted in the use of vasopressors. This composite cardiovascular outcome was chosen because, in this MIS-C cohort,6 low LVEF did not always result in the use of vasopressors and distributive shock was not always associated with low LVEF; however, either finding would influence therapy decisions. Outcomes were measured on or after day 2 to allow for a clinical response after initial treatments.6 Secondary outcomes were the individual components of the primary outcome, the receipt of adjunctive immunomodulatory treatment on or after day 1, persistent or recurrent fever (body temperature, >38.0°C) on or after day 2, and length of stay in the ICU (counted starting from the day of initial treatment [day 0]). Data on treatment-related adverse events were not collected as part of the registry.
STATISTICAL ANALYSIS
Continuous variables were expressed as medians and interquartile ranges or ranges, and categorical variables were expressed as counts and percentages. To assess the associations between initial treatment with IVIG plus glucocorticoids, or with IVIG alone, and the primary and secondary outcomes, we used propensity-score matching and an inverse-probability-weighted analysis, with adjustment for confounding factors that might have influenced treatment choices.20
We modeled the probability of treatment using logistic regression and used the estimated probability as a propensity score. We included relevant baseline variables that might have affected treatment decisions. These included demographic characteristics (age, race or ethnic group, and sex), preexisting conditions, commonly measured laboratory markers of inflammation on the day of admission (neutrophil-to-lymphocyte ratio, C-reactive protein level, and platelet count), and clinical observations at admission (Kawasaki’s disease–like features [as defined by American Heart Association guidelines7], severe cardiovascular or respiratory involvement, vasopressor use, receipt of mechanical ventilation, pulmonary infiltrates on radiographic imaging, and admission to the ICU). Variables were selected on the basis of clinical experience,1,21,22 review of paired correlations, and the success of balancing distributions of treatment groups. We excluded patients who had missing laboratory test results, and we performed a complete-case analysis after covariates were selected.
In the propensity-score–matched analysis, we used “greedy nearest-neighbor” matching without replacement. Here, the matching algorithm first selected a patient who had received IVIG plus glucocorticoids and then selected a patient who had received IVIG alone who had a linear propensity score that was closest to that of the first selected patient. We used a 1:1 ratio within a caliper width of 0.2 of the standard deviation of the logit of the propensity score. Risk ratios and 95% confidence intervals were calculated to quantify the association between the treatment and outcome with the use of log-binomial regression models. We adjusted for treatment site in the propensity-score–matched analysis using generalized estimating equations clustered according to treatment site with an exchangeable correlation structure. We then evaluated the potential effectiveness of treatment on the primary and secondary outcomes using an inverse-probability-weighted analysis to maintain all members of the treatment-comparison cohort by assigning each patient a weight that was based on the propensity score. We used the matching package from R software, version 4.0.2, for the propensity-score–matched analysis and SAS software, version 9.4, for the inverse-probability-weighted analysis.