We present five cases of severe venous thromboembolism in unusual sites and concomitant thrombocytopenia that occurred 7 to 10 days after vaccination for Covid-19. Four of the patients had severe cerebral venous thrombosis with intracranial hemorrhage, and the outcome was fatal in three. Thrombotic thrombocytopenic purpura and immune thrombocytopenic purpura were not suspected because of the absence of hemolysis and because of the good response to platelet transfusions, respectively. A common denominator in all five patients was a high level of antibodies to PF4–polyanion complexes. We therefore propose that these cases represent a vaccine-related variant of spontaneous heparin-induced thrombocytopenia that we refer to as vaccine-induced immune thrombotic thrombocytopenia (VITT).
All the patients had strikingly high levels of antibodies to PF4–polyanion complexes. Although the optical density values may not be directly comparable between studies, it is worth noting that 5 to 7% of blood donors have detectable PF4–heparin antibodies; however, typical blood donors rarely have levels yielding an optical density higher than 1.6.7 Moreover, in patients with typical heparin-induced thrombocytopenia, optical density values higher than 2 are unusual.8 Nearly all healthy adults have a reservoir of B cells specific for PF4–heparin complexes; production of “heparin-induced thrombocytopenia–like” antibodies by these B cells is kept in check by immune regulatory mechanisms.9,10
In contrast to platelet aggregation in patients with typical heparin-induced thrombocytopenia, platelet aggregation in our patients was less dependent on physiological levels of heparin and was less sensitive to inhibition with high-dose heparin. Since Patients 1, 2, 3, and 4 had received low-molecular-weight heparin before blood samples were obtained, we cannot rule out the presence of circulating complexes containing antibodies bound to PF4 and low-molecular-weight heparin. Such complexes are generally disrupted in the presence of a high concentration of unfractionated heparin (96 IU per milliliter), but this was not observed in all cases. Moreover, Patient 5 had not received heparin. Collectively, these results suggest that the serum in these patients contained immune complexes with a mixture of antibody specificities similar to those described in the serum of patients with autoimmune heparin-induced thrombocytopenia.8 It has not yet been determined whether the serum in our patients contained antibodies that bound PF4 alone.
Our findings indicate a shared pathophysiological basis of the condition in these five patients and should raise awareness that a syndrome similar to autoimmune heparin-induced thrombocytopenia may occur in some persons after vaccination with ChAdOx1 nCoV-19. By providing a link between thrombosis and the immune system, these results strengthen the view that vaccination may have triggered the syndrome.
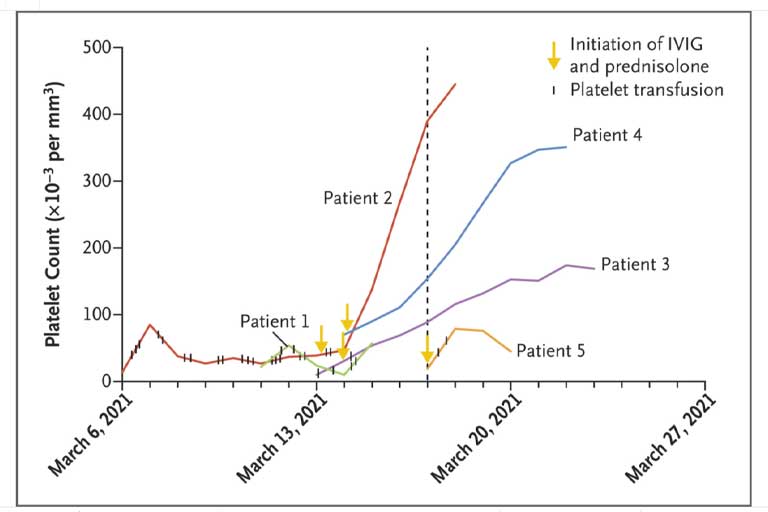
In these cases, the characteristic antibodies were first identified after the initiation of anticoagulation treatment with low-molecular-weight heparin for life-threatening thrombosis and thrombocytopenia (Figure 1). With the antibody results in hand, the clinicians faced the dilemma of deciding which anticoagulant to administer during this syndrome, which is usually associated with heparin. However, platelet counts were increasing after concomitant treatment with intravenous immune globulin and prednisolone had been initiated, and no clinical evidence suggested that thrombosis was increasing. Moreover, there were significant concerns that administration of anticoagulation alternatives to heparin or low-molecular-weight heparin might lead to aggravation of the ongoing intracerebral hemorrhage. Fondaparinux has a longer half-life than low-molecular-weight heparin, and a well-documented reversal strategy for factor Xa inhibitors is not available. It is worth noting that platelet counts continued to increase in all the patients despite continuation of treatment with low-molecular-weight heparin (Figure 1). This finding may reflect the efficacy of early treatment with intravenous immune globulin, which has proved to be highly effective against spontaneous heparin-induced thrombocytopenia.11
Treating severely ill patients such as those described in this report is always challenging. The most important implication of our findings is that physicians should have a low threshold for requesting ELISA testing for PF4–polyanion antibodies, including confirmatory functional testing, in patients who have unexpected symptoms after vaccination.
Although rare, VITT is a new phenomenon with devastating effects for otherwise healthy young adults and requires a thorough risk–benefit analysis. The findings of our study indicate that VITT may be more frequent than has been found in previous studies in which the safety of the ChAdOx1 nCoV-19 vaccine has been investigated.12