En atención a la creciente preocupación sobre la confianza en...
Leer más
Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine
The efficacy of two injections of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike messenger RNA (mRNA) vaccines (BNT162b2 [Pfizer] and mRNA-1273 [Moderna])1 in preventing symptomatic SARS-CoV-2 infection in persons without previous coronavirus disease 2019 (Covid-19) has been shown to be high. We wondered what the response would be to the first vaccine dose in persons with previous Covid-19.
We took advantage of our ongoing institutional review board–approved, longitudinal PARIS (Protection Associated with Rapid Immunity to SARS-CoV-2) study to provide a limited snapshot of the antibody responses in 110 study participants with or without documented preexisting SARS-CoV-2 immunity (mean age overall, 40.0 years [range, 24 to 68; ≥60 years, 8%]; 67 seronegative participants [64% female] with a mean age of 41.3 years and 43 seropositive participants [59% female] with a mean age of 41.4 years) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org) who received their first spike mRNA vaccine dose in 2020 (88 received the Pfizer vaccine and 22 the Moderna vaccine). SARS-CoV-2 spike IgG was measured with the use of a previously described two-step enzyme-linked immunosorbent assay and expressed as area under the curve (AUC).
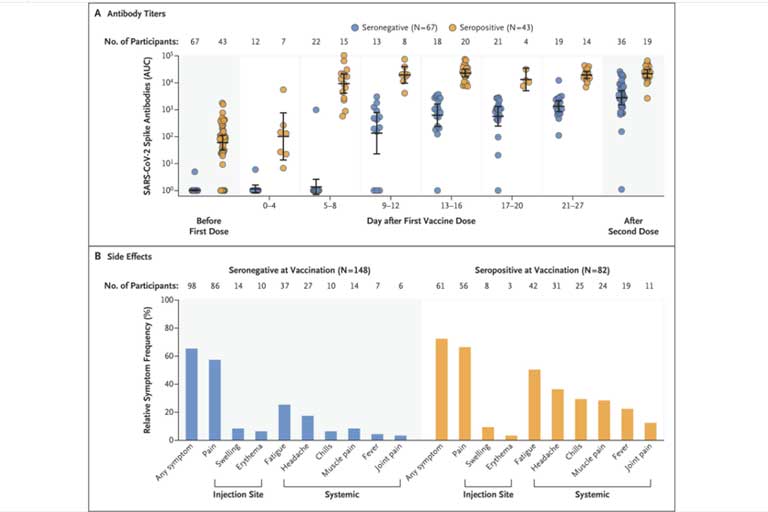
Repeated sampling after the first dose indicates that the majority of seronegative participants had variable and relatively low SARS-CoV-2 IgG responses within 9 to 12 days after vaccination (median AUC before vaccination, 1 [67 participants]; at 0 to 4 days, 1 [12 participants]; at 5 to 8 days, 1 [22 participants]; at 9 to 12 days, 439 [13 participants]; at 13 to 16 days, 1016 [18 participants]; at 17 to 20 days, 1037 [21 participants]; at 21 to 27 days, 1293 [19 participants]; and after the second dose, 3316 [36 participants]). In contrast, participants with SARS-CoV-2 antibodies at baseline before the first vaccine injection rapidly developed uniform, high antibody titers within days after vaccination (median AUC before vaccination, 90 [43 participants]; at 0 to 4 days, 133 [7 participants]; at 5 to 8 days, 14,208 [15 participants]; at 9 to 12 days, 20,783 [8 participants]; at 13 to 16 days, 25,927 [20 participants]; at 17 to 20 days, 11,755 [4 participants]; at 21 to 27 days, 19,534 [14 participants]; and after the second dose, 22,509 [19 participants]).
The antibody titers of vaccinees with preexisting immunity were 10 to 45 times as high as those of vaccinees without preexisting immunity at the same time points after the first vaccine dose (e.g., 25 times as high at 13 to 16 days) and also exceeded the median antibody titers measured in participants without preexisting immunity after the second vaccine dose by more than a factor of 6. Although the antibody titers of the vaccinees without preexisting immunity increased by a factor of 3 after the second vaccine dose, no increase in antibody titers was observed in the Covid-19 survivors who received the second vaccine dose. No substantial difference was noted in the dynamics of antibody responses elicited by the Pfizer and Moderna vaccines after the first dose (Fig. S1). The current analysis represents a convenience sample in which not all participants were able to provide biospecimens for antibody analysis at all the additional time intervals. Ongoing follow-up studies will show whether these early differences in immune responses are maintained over a prolonged time period.
In addition, we compared the frequency of local, injection-site–related as well as systemic reactions after the first dose of vaccine in 230 participants (mean age, 39.2 years [range, 22 to 70; ≥60 years, 8%]; 148 seronegative participants [70% female] and 82 seropositive participants [64% female]). Overall, both vaccines (156 participants received the Pfizer vaccine and 74 the Moderna vaccine) had no side effects that resulted in hospitalization. A total of 159 of the 230 participants (69%) who completed the PARIS study survey reported having some side effects after the first vaccine dose (46% of the seronegative survey respondents and 89% of the seropositive survey respondents). Most common were localized injection-site symptoms (pain, swelling, and erythema), which occurred with equal frequency independently of the serostatus at the time of vaccination and resolved spontaneously within days after vaccination. Vaccine recipients with preexisting immunity had systemic side effects at higher frequencies than those without preexisting immunity (fatigue, headache, chills, muscle pain, fever, and joint pain, in order of decreasing frequency). Because a convenience sample was used and only participants with available data were studied, caution is needed until the full data set, including side effects occurring after the first as well as the second vaccine dose, can be assessed.
We found that a single dose of mRNA vaccine elicited rapid immune responses in seropositive participants, with postvaccination antibody titers that were similar to or exceeded titers found in seronegative participants who received two vaccinations. Whether a single dose of mRNA vaccine provides effective protection in seropositive persons requires investigation.
Florian Krammer, Ph.D.
Komal Srivastava, M.S.
Hala Alshammary, M.S.
Angela A. Amoako, M.S.
Mahmoud H. Awawda, M.S.
Katherine F. Beach, B.S.
Maria C. Bermúdez-González, M.P.H.
Dominika A. Bielak, B.A.
Juan M. Carreño, Ph.D.
Rachel L. Chernet, B.A.
Lily Q. Eaker, B.A.
Emily D. Ferreri, B.S.
Daniel L. Floda, B.A.
Charles R. Gleason, B.S.
Joshua Z. Hamburger, M.D.
Kaijun Jiang, M.S.
Giulio Kleiner, Ph.D.
Denise Jurczyszak, Ph.D.
Julia C. Matthews, B.A.
Wanni A. Mendez, A.A.S.
Ismail Nabeel, M.D.
Lubbertus C.F. Mulder, Ph.D.
Ariel J. Raskin, B.A.
Kayla T. Russo, B.S.
Ashley-Beathrese T. Salimbangon, B.A.
Miti Saksena, M.B., B.S.
Amber S. Shin, B.S.
Gagandeep Singh, Ph.D.
Levy A. Sominsky, B.A.
Daniel Stadlbauer, Ph.D.
Ania Wajnberg, M.D.
Viviana Simon, M.D., Ph.D.
Icahn School of Medicine at Mount Sinai, New York, NY
florian.krammer@mssm.edu, viviana.simon@mssm.edu
Supported by the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Influenza Vaccine Innovation Centers (contract 75N93019C00051), the NIAID Centers of Excellence for Influenza Research and Surveillance (contract HHSN272201400008C), the JPB Foundation, the Open Philanthropy Project (research grant 2020-215611 [5384]), and anonymous donors.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Créditos: Comité científico Covid




